Direct Amp Influenza AB Kit (RUO)
The A2K Influenza A/B Multiplex Assay is a real-time reverse-transcription polymerase chain reaction (rRT-PCR) laboratory test that can simultaneously detect and differentiate between influenza A and influenza B in upper or lower respiratory specimens. Combine the RT-PCR assay with the powerful A2K Direct amplification mastermix for reliable detection of Influenza in crude sample - No extraction required.

Kit Contents
- Influenza AB RT-PCR multiplex assay
- DirectAmp Mastermix
- DirectAmp Enhancer
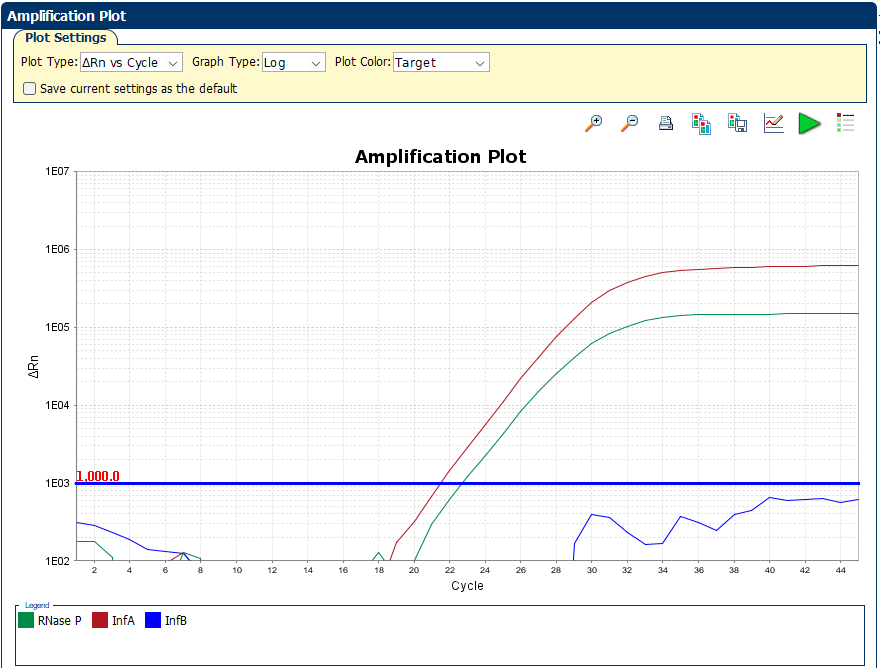
Features
✓ 1000/5000 reactions per kit
✓ Targeted up to 100% of currently available complete genomes for Influenza A & B
✓ Primers and probes selected from an evolutionarily well conserved regions of the M1 & NS2 genes
✓ Targeted RNAse P gene for internal control and collection efficacy monitor
✓ Identical in sequence to the CDC Influenza SARS-CoV-2 EUA
Technical Specifications*
-
Module 1
- Influenza A
- Influenza B
- RNAse P
-
A2K Evaluation
✓ Analytical Controls
✓ Whole genome standards
✓ Clinical Nasopharyngeal Swabs
✓ Crude or Purified samples
-
Multiplex Channels
- FAM
- YAK
- Cy5